Possible Link between Biological and Industrial Nitrogen Fixation
Martin Kirk
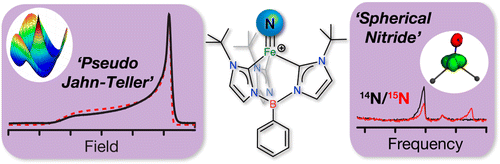
Nitrogen is an essential nutrient that is abundantly available in Earth's atmosphere in the form of the dinitrogen (N2) molecule, which cannot be assimilated by plants and animals. Instead, living things must obtain their nitrogen through "nitrogen fixation", a process in which the very strong and nonpolar N≡N triple bond is cleaved to generate two ammonia (NH3) molecules. This biological process occurs through the action of the enzyme nitrogenase, found only in a select group of microorganisms. In industry, nitrogen fixation is achieved through the Haber-Bosch process, which celebrated its centenary last year. Now Jeremy Smith, Martin Kirk, Brian Hoffman, and co-workers have characterized a new iron-nitride complex that provides a possible link between these two processes (DOI: 10.1021/ja505403j).
The team combines advanced electron-nuclear double resonance (ENDOR) spectroscopic measurements with quantum chemical calculations to characterize a Jahn-Teller distortion in which the iron-nitride complex decreases its energy by lowering its symmetry. Surprisingly, the investigation further reveals an essentially spherical nitride trianion bound to iron. The results suggest an orbital selection mechanism for proton or H-atom transfer with significant implications for the catalytic conversion of N2 to NH3 by nitrogenase and industrial catalysts.
