News & Events
MSC03 2060
300 Terrace St. NE
Albuquerque, NM 87131-0001
Physical Location:
Clark Hall
Phone: 505-277-6655
chemistry@unm.edu
MSC03 2060
300 Terrace St. NE
Albuquerque, NM 87131-0001
Physical Location:
Clark Hall
Phone: 505-277-6655
chemistry@unm.edu

Profile: Dr. Shi obtained his BS and MS in Chemistry from the University of Nankai University, China in 1994 and 1997, respectively. He received his Ph.D. in Chemistry at the University of Maryland, College Park, MD. His mentor was Professor Jeffery T. Davis. Dr. Shi was a Postdoc Research Associate in the Department of Chemistry at the University of California Berkeley under Professor Paul Bartlett and Professor F. Dean Toste from 2002 to 2005. Dr. Shi was a Research Scientist at General Electric in 2005. He was an Assistant, Associate, and then Full Professor in the Department of Chemistry at West Virginia University beginning in 2005 until 2015. In 2015, he became an Associate Professor in the Department of Chemistry at the University of South Florida. In 2017 Dr. Shi became a Professor in the Chemistry Department at the University of South Florida where is currently working today.
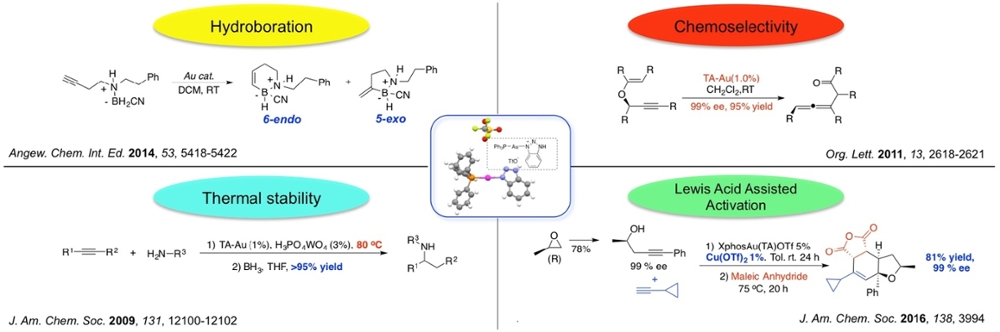
Abstract: Homogeneous gold catalysis has been developed explosively during the past decades. Despite the remarkable electrophilic activation of alkynes by cationic Au(I) catalysts, such as PPh3Au+, one challenge is to overcome their poor stability at high temperature. However, in order to activate some less reactive substrates, such as internal alkynes, harsher reaction conditions are usually required.
As a good σ-donor and π-receptor, triazole has been applied as a ligand to improve the stability of cationic Au(I) catalysts. Taking advantage of the good stability of triazole-Au(I) complexes (TA-Au), we successfully achieved good reactivity of intermolecular hydroamination for both terminal and internal alkynes. Unlike previous reported gold catalysts, the TA-Au catalysts activate alkynes selectively over allenes. With this excellent chemoselectivity, TA-Au catalysts showed interesting reactivity in propargyl ester and vinyl ether rearrangement. This facilitates the development of otherwise challenging transformations, for instance, asymmetric synthesis of substituted allenes, Schmittel cyclization, dienal synthesis, and so on.
References
See Announcement