News & Events
MSC03 2060
300 Terrace St. NE
Albuquerque, NM 87131-0001
Physical Location:
Clark Hall
Phone: 505-277-6655
chemistry@unm.edu
MSC03 2060
300 Terrace St. NE
Albuquerque, NM 87131-0001
Physical Location:
Clark Hall
Phone: 505-277-6655
chemistry@unm.edu

Profile: Dr. Cynthia J. Burrows is the Thatcher Distinguished Professor of Chemistry at the University of Utah and presently Chair of the Department of Chemistry. She was raised in St. Paul, Minnesota and Boulder, Colorado. Her early training was in physical organic chemistry with Prof. Stan Cristol at the University of Colorado (B. A. 1975) and Prof. Barry Carpenter at Cornell University (Ph.D., 1982), followed by a NSF-CNRS postdoctoral fellowship in the laboratory of Prof. Jean-Marie Lehn, Université Louis Pasteur, Strasbourg (1981-83). From 1983-1995, she was on the faculty at the Stony Brook University, before returning to the West to take a position at the University of Utah in Salt Lake City in 1995.
The Burrows research group investigates the chemistry and biochemistry of modified bases in DNA and RNA with a focus on oxidative stress, an underlying component of age-related diseases such as cancer. The approach is multi-disciplinary involving the organic chemistry of base modification in DNA, the enzymology of polymerase bypass and DNA repair, biophysical studies of the effects of base modification, single-molecule studies of DNA and RNA in nanopores, whole-genome sequencing and cellular studies of synthetically modified oligomers.
Prof. Burrows served as Senior Editor of the Journal of Organic Chemistry for many years and since January 2014 is Editor-in-Chief of Accounts of Chemical Research. She is a past recipient of the Robert Parry Teaching Award and the University Distinguished Teaching Award; her research was recognized with the ACS Utah Award, ACS Cope Scholar Award, and the University of Utah’s Distinguished Creative and Scholarly Research Award; she is also the 2018 recipient of the James Flack Norris Award in Physical Organic Chemistry. She was inducted into the American Academy of Arts and Sciences in 2009 and elected to the National Academy of Sciences in 2014.
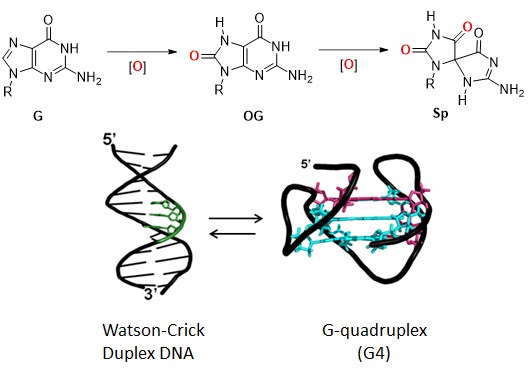
Abstract: Less than 2% of the human genome codes for the amino acid sequence of proteins. Why is all the rest of the DNA there? Some of it participates in orchestrating replication, some in the protection of the ends (telomeres), and some sequences upstream of a gene (promoters) control whether or not a gene is expressed as protein. All of these functions of DNA can involve guanine-rich sequences capable of folding into G-quadruplexes—four-stranded folds of DNA that differ dramatically from the classical base-pairing scheme of the Watson-Crick double helix. Furthermore, the G-rich sequences are sensitive to oxidative stress, converting to modified structures including 8-oxo-7,8-dihydroguanine (OG) and hyperoxidized lesions (Sp). Both the overall reactivity of a G residue in DNA or RNA and the final oxidized G product formed are highly dependent on sequence, solvent exposure and mechanism. For example, oxidation of G in G-quadruplex folds leads to very different outcomes compared to those in Watson-Crick B-helical duplexes. The location of G damage in turn has a profound effect on the stability of duplex vs. quadruplex structures. We propose that G-rich sequences respond to oxidative stress by selecting a secondary structure that can best accommodate the damaged base, and that ‘shape-shifting’ may be used as a signaling mechanism to affect transcription and repair. The implications are that nucleotide identity beyond the exome may be important in gene expression and disease, and that the definition of epigenetic modifications should be expanded to include guanine oxidation.
See Announcement